OncoMate® MSI Dx Analysis System
- CE-marked and available in select European countries; FDA-approved in the United States
- Fragment sizing test used to determine MSI status with Gold Standard microsatellite markers
- First and only MSI by PCR test FDA-approved in the US for companion diagnostic use in endometrial carcinoma for use in your lab
Catalog Number:
Size
Catalog Number: MD3140
Accurately Determine MSI Status of Tumors Using the Gold Standard Method
The OncoMate® MSI Dx Analysis System is a fluorescent, multiplex PCR-based test to detect microsatellite instability (MSI). MSI is a form of genomic instability caused by the insertion or deletion of repeating bases within microsatellites during DNA replication due to the failure of the mismatch repair system (MMR) to correct these errors.
The OncoMate® MSI Dx Analysis System (Cat.# MD3140) is available in the following countries: Austria, Belgium, Czech Republic, Denmark, France, Germany, Hungary, Ireland, Italy, Luxembourg, Netherlands, Norway, Poland, Romania, Slovakia, Spain, Sweden, Switzerland and the United Kingdom. For information about availability in other regions, including the FDA-cleared product in the United States, please contact us.

Patients with MSI-H Tumors are More Likely to Respond to Immune Checkpoint Inhibitor Therapies
MSI-H tumors express protein neoantigens that can result in lymphocyte infiltration. Some tumors can block immune activation through the expression of PD-L1. This tumor induced inhibition of immune cell activity can be overcome with immune checkpoint inhibitor (ICI) therapies, which enhance the immune response against tumor cells and generally improve a patient’s response to these treatments1–3.
Better Information to Inform Better Treatment Options
The MSI gold standard research tool is now an IVD, enabling better access for clinical diagnostics laboratories. The OncoMate® MSI Dx Analysis System is a CE-Marked IVD Medical Device, leveraging the same informative MSI loci relied on by global clinical researchers for almost two decades. The improved system is designed for use as a diagnostic with cancer patients to better inform testing and treatment options.
In a retrospective study of colorectal cancers, the OncoMate® MSI Dx Analysis System showed concordance with sequencing and immunohistochemistry4 and is intended to identify patients that may benefit from further diagnostic testing.
Only Two Sections Required

FFPE sections with a tissue volume of 0.1mm3 to 2.0mm3. Tumor sample should have >20% viable tumor cells.
Minimal DNA Required

Uses only 1ng of amplification-quality DNA per reaction.
Assay Time Under 3 Hours

Quick, multiplexed reaction, balanced for accuracy and efficiency, goes from DNA to answer in as little as 2.5 hours.
Sensitive and Specific Results

Reliable MSI determination with strong agreement with IHC.
Test Design and Marker Information
The OncoMate® MSI Dx Analysis System targets five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) that were selected for high sensitivity and specificity to alterations in repeat lengths in samples containing mismatch repair defects5,6. For quality control and sample authentication of matched tumor and normal samples, the system uses two pentanucleotide repeat markers (Penta C and Penta D) that were selected for their high level of polymorphism and lower degree of MSI.
Biomarker and Loci Information
Target Markers

NCI recommended, Revised Bethesda Panel and two pentanucleotide markers7.
Example Data
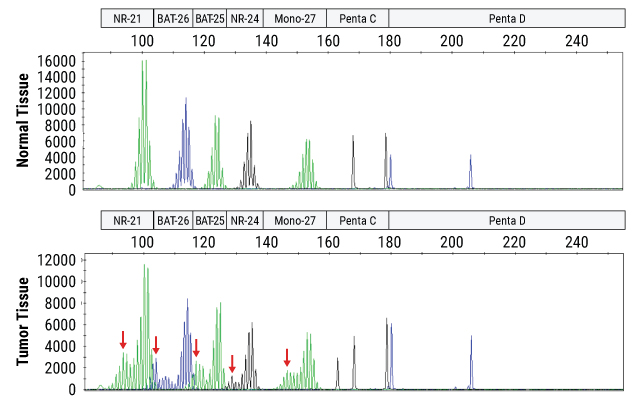
Instability is determined by fragment size analysis on a capillary electrophoresis instrument following PCR amplification of DNA from a patient's normal and tumor tissue samples. Unstable loci in the tumor tissue are indicated by red arrows.
Tumor to Answer Overnight
The OncoMate® MSI Dx Analysis System is part of a broader workflow that includes DNA extraction from FFPE tissue samples, quantitation of DNA, amplification of specific microsatellite markers using multiplex PCR, fragment separation by capillary electrophoresis, and data analysis and interpretation.

Isolate DNA
from FFPE samples

Quantitate DNA
using fluorescent DNA quantitation reagents and instruments

Amplify DNA
using the OncoMate® MSI Dx Analysis System

Calibrate the Dye Spectrum
using the OncoMate® 5C Matrix Standards

Separate and Detect Fragments
using a capillary electrophoresis instrument capable of fluorescent detection

Data Analysis and Reporting
using fragment analysis software

Turnaround Time:
In as little as 10 hours or overnight

Overview of the OncoMate® MSI Dx Analysis System Workflow

Compatibility Specifications
Specimens
Normal and tumor FFPE tissue samples with a volume of 0.1–2.0mm3. Tumor samples must contain at least 20% viable tumor cells.
DNA Quantity and Quality
Uses a target of 1ng per reaction, as estimated by a dsDNA binding dye or a qPCR system. These methods provide a reliable estimate of DNA concentration with highly fragmented DNA like that purified from FFPE.
Thermal Cycling
Use a thermal cycler that allows block ramp rates from 3.9°C to 5.0°C per second.
Data Analysis Software
Label (i.e., color), tabulate and display data from a mixture of fragment sizes (65bp to 300bp) and colors (5), and print and export analyzed data according to calculated allele size and marker.
Spectral Calibration
Prior to first use, perform a spectral calibration of the capillary electrophoresis instrument using the OncoMate® 5C Matrix Standard.
Capillary Electrophoresis
Use with capillary electrophoresis instruments capable of:
Fragment resolution of 1bp from 60bp to ≥300bp
Sizing precision ≤0.15bp across a range from 60bp to ≥300bp.
Calibration and detection of 5 channel collection with excitation wavelengths from 480nm to 520nm and detection optics to capture from approx 500nm to 630nm.
Fragment Analysis Marker Panels and Settings
Capillary electrophoresis data requires further analysis to assign marker and size information. We have provided fragment analysis marker panels and analysis settings for download. The provided settings were used during the performance evaluation of the OncoMate® MSI Dx Analysis System and may required optimization for your chosen capillary electrophoresis equipment.
Intended Use Statement: The OncoMate® MSI Dx Analysis System is a PCR-based fragment-sizing test used to determine microsatellite instability (MSI) status in DNA purified from human formalin-fixed paraffin-embedded (FFPE) tissue samples derived from solid tumors.
The OncoMate® MSI Dx Analysis System generates allelic profiles from tumor and non-tumor FFPE tissue samples from the same patient through polymerase chain reaction (PCR) amplification of DNA microsatellite markers, followed by size separation of the amplified markers using capillary electrophoresis. MSI status is determined by comparing the allelic profiles. An expansion or reduction in the length of repetitive DNA sequences in the tumor cell DNA when compared to the normal cell DNA from the same patient indicates MSI. Normal and tumor tissue from the same patient must be tested at the same time and data from both samples must be available for comparison for results to be valid.
The OncoMate® MSI Dx Analysis System is not intended to diagnose a specific disease. It is intended for use with patients already diagnosed with cancer who may benefit from additional genetic testing. Test results obtained using the product must be interpreted by healthcare professionals in conjunction with other clinical findings, family history and laboratory data. This product is intended for professional use only.
Comparative Performance of Methods to Detect MSI in Endometrial Tumors
A recent paper published by researchers in Italy compared the performance of detecting MSI in endometrial samples using four molecular tests including capillary electrophoresis (OncoMate® MSI, Promega), microcapillary electrophoresis (TapeStation 4200, Agilent), and high-resolution (HRM) analysis approaches (Idylla™ MSI Test, Biocartis; EasyPGX® ready MSI, Diatech Pharmacogenetics). Across the four methods, OncoMate® MSI demonstrated the highest concordance with IHC results and best sensitivity for dMMR tumors.8

The authors note that for endometrial samples MSI detection should rely “…on fluorescent capillary electrophoresis because its resolution is able to identify….critical cases that could be misdiagnosed with other strategies”.
Why Order MSI Testing?
Because MSI is highly correlated with Lynch syndrome, and more recently has been associated with response to immune checkpoint inhibitor (ICI) therapeutics, several professional organizations recommend analysis of MSI and/or DNA mismatch repair protein expression (dMMR by IHC) for many different cancer types 9-14.
Clinical oncology associations that endorse universal screening of colorectal and endometrial cancers for MSI to identify candidates for further diagnostic testing for Lynch Syndrome include the European Society for Medical Oncology (ESMO), the National Comprehensive Cancer Network, and the American Society of Clinical Oncology9,10,14.
EMSO recognizes the importance of MSI and dMMR testing to support patient eligibility for ICI therapies. The recommendations surrounding MSI testing for this purpose have been summarized in the literature13,14.
The ESMO Translational Research and Precision Medicine Working Group recommends MSI testing on the spectrum of Lynch Syndrome tumors because of the potential usefulness of ICI in these cancers and because the identification of Lynch Syndrome can benefit extended family members. They recommend using MSI by PCR and dMMR by IHC together.
Important MSI Publications and Guidelines
Microsatellite instability has become an increasingly relevant tool in genetic and immuno-oncology research. Deficiencies in DNA mismatch repair (MMR) can be caused by hereditary, germline mutations or hypermethylation. Either mechanism disrupts expression of functional MMR proteins, allowing replication errors to accumulate across the genome. Global genomic mutations disrupt normal cellular function, which leads to unchecked growth and cancers but also produces novel proteins. These “foreign” proteins can be immunogenic, recruiting immune effector cells to that tissue. Instability, or mutations, of mononucleotide repeat microsatellite sequences are particularly sensitive to replication errors and can be the first evidence of an MMR deficiency.
Luchini, C. et al. (2019) ESMO recommendations on microsatellite instability testing foe immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumor mutational burden: a systematic review-based approach. Ann Oncol. 30.1232–1243.
Stjepanovic, N. et al. (2019) Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 30,1558–1571.
Fuchs, C.S. et al. (2018) Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncology 10;4(5):e180013. Epub 2018 May 10. Erratum in: JAMA Oncology. 2019 ;1;5:579.
Le, D.T. et al. (2017) Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 10.1126/science.aan6733.
Overman, N.J. et al. (2017) Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18,1182–1191.
Sepulveda, A.R. et al. (2017) Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 35, 1453–86.
Le, D.T. et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New Engl. J. Med. 372, 2509–20.
Boland, C.R. et al. (2010) Microsatellite Instability in Colorectal Cancer. Gastroenterology 138, 2073–87.
Murphy, K.M. et al. (2006) Comparison of the Microsatellite Instability Analysis System and the Bethesda Panel for the Determination of Microsatellite Instability in Colorectal Cancers. J. Mol. Diagn. 8, 305–11.
Umar A. et al. (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 96, 261-8.
Bacher, J.W. et al. (2004) Development of a Fluorescent Multiplex Assay for Detection of MSI-High Tumors. Disease markers 20, 237–250.
References
- Mandal, R. et al. (2019) Science 364, 485–91.
- Dudley, J.C. et al. (2016) Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. 22, 813–820.
- Kok, M. et al. (2019) How I treat MSI cancers with advanced disease. ESMO Open 2019;4:e000511. doi:10.1136/esmoopen-2019-000511.
- OncoMate® MSI Dx Analysis System Performance Characteristics Flyer #FL641I (2020) Promega Corporation.
- Bacher, J. et al. (2004) Dis. Markers 20, 237.
- Luchini, C. et al. (2019) Annals of Oncology 30, 1232.
- Umar, A. et al. (2004) J. Natl. Cancer Inst. 96, 261–8.
- Libera, L. et al. (2022) Hum. Pathol. 128, 134–140.
- National Comprehensive Cancer Network (www.nccn.org). Accessed May 10, 2021.
- Sepulveda, A.R. et al. (2017) J. Mol. Diag. 19, 187–225.
- Le, D.T. et al. (2015) New Engl. J. Med. 372, 2509–20.
- Le, D.T. et al. (2017) Science 10.1126/science.aan6733.
- Luchini, C. et al. (2019) Ann. Oncol. Published online May 6, 2019.
- Stjepanovic, N. et al. (2019) Ann. Oncol. 30, 1558–71.
Seamless Support for Adopting OncoMate® MSI Dx in Your Lab
Our team is dedicated to partnering with you to ensure success and has broad experience training laboratories. We offer an online user training course as well as virtual and in-person training to get your lab up and running with the OncoMate® MSI Dx Analysis System more efficiently.

Medical Information
If you need assistance or access to medical information and resources, our Medical Affairs scientists are ready to help.
Protocols
Specifications
Catalog Number:
What's in the box
| Item | Part # | Choose a size | Concentration |
|---|---|---|---|
Water, Amplification Grade |
MD193A | 1 × 1,250μl | |
OncoMate® MSI 5X Master Mix |
MD280A | 1 × 200μl | |
Size Standard 500 |
MD500A | 1 × 100μl | |
OncoMate® MSI 5X Primer Mix |
MD705A | 1 × 200μl | |
2800M Control DNA |
MD810A | 1 × 25μl | 10ng/μl |
SDS
Search for SDSCertificate of Analysis
Use Restrictions
For In Vitro Diagnostic Use. This product is only available in certain countries.Storage Conditions
Related Products
Frequently Used With
OncoMate® 5C Matrix Standard
Used to calibrate capillary electrophoresis instruments prior to running the OncoMate® MSI Dx Analysis System.
MD3850
Maxwell® CSC Instrument
Benchtop instrument for automated nucleic acid purification from a range of clinical sample types. For In Vitro Diagnostic Use.
AS6000
Maxwell® CSC DNA FFPE Kit
Isolates DNA from FFPE samples for in vitro diagnostic assays using the Maxwell® CSC Instrument.
AS1350
Maxwell® CSC Blood DNA Kit
Isolates DNA from blood for in vitro diagnostic assays using the Maxwell® CSC Instrument.
AS1321

