Clinical Assay Development Services
Bring your assays to market faster. From design and development to manufacturing of high-quality IVD assays, our Clinical Assay Development Services (CADS) provide a seamless delivery pipeline. We provide you with a superior assay development service backed by rigorous quality control, proven technical expertise and our own trusted reagents and technologies.
With CADS, you will benefit from:
- Accelerated time to market
- Deep knowledge of core technologies
- In-house control of supply chain and quality
- Scientist-to-scientist collaboration
- Range of development capabilities
- Mature design control and documentation
Flexible Solutions and Comprehensive Support for Clinical Assay Development
Other assay development providers rely on outside resources to deliver your ideas, slowing progress and delaying critical workflows. Our Clinical Assay Development Services (CADS) combines proven expertise with adaptable technology to help you move from concept through manufacturing with confidence. Simplify with a single partner, from beginning to end. Learn more about our Immunoassay development options below.
Wondering how to choose the right tool for your clinical immunoassay development?
Our Top 5 Reasons to Modernize Your Immunoassay Detection Tools
Hear from a Scientist
Watch our interview with Senior Research Scientist, Dr. Melanie Dart, where we discuss the impact of bioluminescent detection on immunoassay performance and more.
How Lumit® Flex Technology Works
Lumit® Flex is a bioluminescent immunoassay system that detects specific antigens or antibodies using a patented Add-Mix-Read process. The system employs two small peptide tags and a detection substrate that works as follows:
- Peptide α and Peptide β are attached to assay antibodies or antigens.
- When both peptides bind the same target, the peptide tags move into close proximity.
- The Detection Substrate reassembles into an active NanoLuc® luciferase, producing a light signal that directly reflects analyte concentration.

Lumit® Flex System simplifies immunoassays with two small peptide tags and a detection protein.

Single Samples

Low-to-Mid Throughputs

High-Throughput

We offer a uniquely comprehensive portfolio of technology development, manufacturing and logistics that enable us to support every step of your clinical assay development, from conception to delivery.

Faster results: Complete assays in as little as 5–90 minutes.
Simplified workflow: Homogeneous Add-Mix-Read design reduces manual steps and variability.
High sensitivity and dynamic range: Detect low-abundance targets with minimal background noise.
Easy integration: Works with standard luminescence-capable microplate readers—no new equipment required.
Consistent results: No washes or multiple incubation steps.
Scalable performance: Suitable for point-of-care to high-throughput applications.
Stable and reliable: Lyophilization-compatible, all-in-one format stable at room temperature.
Flexible formats: Performs even with difficult targets or challenging sample types, adaptable across sample types, analytes, assay formats, and workflows.
See how Lumit® Flex Technology is used in these peer-reviewed publications:
Kincaid, V. A. et al. (2022) Simple, Rapid Chemical Labeling and Screening of Antibodies with Luminescent Peptides. ACS Chem. Biol. 17, 8, 2179-2187.
Hall, M. P. et al (2021) Toward a Point-of-Need Bioluminescence-Based Immunoassay Utilizing a Complete Shelf-Stable Reagent. Anal. Chem. 93, 12, 5177-5184.
Torio, E. A. et al. (2022) Development of a rapid, simply, and sensitive point-of-care technology platform utilizing ternary NanoLuc. Front. Microbiol., Sec. Infectious Agents and Disease. 13.
Developing custom PCR assays can be challenging. Each stage, from design to formulation, introduces opportunities for variability and delay. Our Clinical Assay Development Services (CADS) streamlines the process with expert support from assay design through manufacturing, helping you reduce development time, minimize troubleshooting, and achieve reliable performance across liquid and lyophilized formats.
How CADS Supports Your PCR Amplification Assay Development
Whether you have defined primers and probes or simply a target of interest, CADS can support your PCR development journey. Our flexible model delivers the level of customization that best fits your resources and goals, providing a fully optimized, ready-to-run assay tailored to your needs.

Lyophilized PCR Amplification Assays
Our lyophilized bead format delivers convenience, stability, and workflow simplicity. Each bead contains all necessary PCR reagents for your assay—just add your sample. This format supports ambient shipping and storage, reduces preparation steps, and helps ensure consistency from run to run.
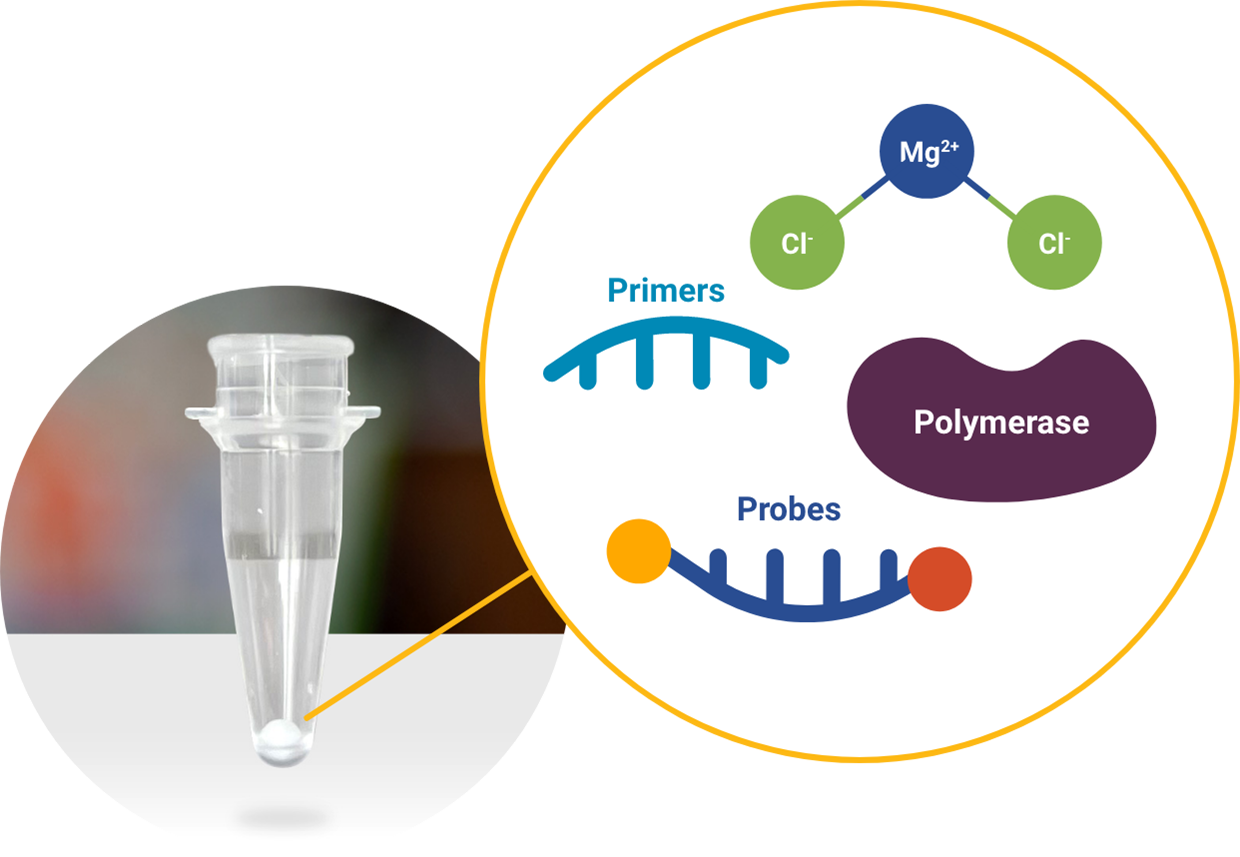
Our lyophilized beads contains all necessary PCR reagents for your assay.
Build Your Own Amplification Assay
We offer flexible PCR master mix components for teams who prefer to build and optimize their clinical assays in-house. Explore our portfolio of preformulated reagents designed for both liquid and lyophilization-compatible workflows. Customize your mix and configure your ideal amplification conditions with our modular kits.
Formats available: Liquid | Lyophilization-compatible liquid | Lyophilized beads

From Our Experts: Designing Trust in Clinical Assay Development
What makes a development partner truly reliable? Explore how trust, scientist-to-scientist collaboration and an integrated pipeline, from concept through manufacturing, can help you bring assays to market with confidence.
Let's Partner to Develop Your Next Assay
Whether you have a project in mind or simply want to get more details about how our capabilities can meet your needs, we welcome the opportunity to develop your next assay.
Manufacturing Capabilities
- Nucleic Acid Chemistry
- Bioluminescence
- Cell Manufacturing
- Protein Purification
- Fermentation
- Custom Manufacturing
- Amplification and Human Identify
- cGMP Contract Manufacturing


Our manufacturing infrastructure features:
- Room to grow for future needs: over 450,000 square feet (41,000m2) of manufacturing space.
- Strong relationships: Over half of our suppliers are located within 50 miles of our Madison, Wisconsin manufacturing facilities.
- Deep knowledge: The average tenure of our manufacturing leaders is 15+ years.
- Exemplary customer experience: surveyed customers have >99% satisfaction rate.
Our rigorous quality system ensures we can develop, manufacture, test and deliver high-quality products around the world. Our global supply chain, product quality, design and quality documentation are managed in-house, providing you greater peace of mind.
To demonstrate our commitment to meeting industry standards, we maintain ISO certifications at all global manufacturing sites.
- ISO 9001:2015 Provides general quality management system requirements
- ISO13485:2016 Provides quality management system requirements for medical devices
- ISO18385:2016 Minimizes the risk of human DNA contamination in products
- MDSAP (Medical Device Single Audit Program)
- ISO 13485:2016
- Canada – Medical Device Regulations – Part 1 – SOR 98/282
- USA – 21 CFR 820, 21 CFR 803, 21 CFR 806, 21 CFR 807 – Subparts A to D

Request a Consult
Whether you have a question about our Clinical Assay Development Services or are ready to get started, we are here to help.
Fill out the form below and a member of our CADS team will be in touch with you shortly.
